Atom Economy Examples
Atom Economy Examples. Let's do some examples of simple reactions. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water
Nejlepší Green Chemistry For Chemical Synthesis Pnas
Hydrogen can be manufactured by reacting methane with steam: In these three examples, reactions 1 to 3: Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Hydrogen can be manufactured by reacting methane with steam:Hydrogen can be manufactured by reacting methane with steam:
May 08, 2012 · this is an example of poor atom economy! Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Continua nella prossima slide ; Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water In these three examples, reactions 1 to 3: Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. May 08, 2012 · this is an example of poor atom economy! Hydrogen can be manufactured by reacting methane with steam:

Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants.. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) C = 12.0, h = 1.0, o = 16.0. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water.. C = 12.0, h = 1.0, o = 16.0.

If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. .. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g)

Continua nella prossima slide ;. In these three examples, reactions 1 to 3: Continua nella prossima slide ; The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. May 08, 2012 · this is an example of poor atom economy! C = 12.0, h = 1.0, o = 16.0.. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.
Let's do some examples of simple reactions... Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Continua nella prossima slide ; Let's do some examples of simple reactions. As shown below this reaction has only 50% atom economy. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. In these three examples, reactions 1 to 3: This idea came together with a desire to be able to measure rationally the created waste and … Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water

Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. In these three examples, reactions 1 to 3: Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Let's do some examples of simple reactions. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. C = 12.0, h = 1.0, o = 16.0. Hydrogen can be manufactured by reacting methane with steam: If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. May 08, 2012 · this is an example of poor atom economy! The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.

May 08, 2012 · this is an example of poor atom economy! May 08, 2012 · this is an example of poor atom economy! Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Continua nella prossima slide ; The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. It is often measured in terms of percent, known as percentage atom economy. Hydrogen can be manufactured by reacting methane with steam: Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.
As shown below this reaction has only 50% atom economy. .. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.

Percentage yield is calculated from the mass of reactants and the mass of products. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. As shown below this reaction has only 50% atom economy. Let's do some examples of simple reactions. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. C = 12.0, h = 1.0, o = 16.0. Percentage yield is calculated from the mass of reactants and the mass of products. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. C = 12.0, h = 1.0, o = 16.0.

Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. C = 12.0, h = 1.0, o = 16.0. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Percentage yield is calculated from the mass of reactants and the mass of products. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Hydrogen can be manufactured by reacting methane with steam: May 08, 2012 · this is an example of poor atom economy! Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. This idea came together with a desire to be able to measure rationally the created waste and … Percentage yield is calculated from the mass of reactants and the mass of products.

Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. As shown below this reaction has only 50% atom economy. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Percentage yield is calculated from the mass of reactants and the mass of products. Let's do some examples of simple reactions. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Hydrogen can be manufactured by reacting methane with steam: Continua nella prossima slide ; Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants.. May 08, 2012 · this is an example of poor atom economy!

Let's do some examples of simple reactions.. This idea came together with a desire to be able to measure rationally the created waste and … May 08, 2012 · this is an example of poor atom economy! The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Hydrogen can be manufactured by reacting methane with steam: If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Percentage yield is calculated from the mass of reactants and the mass of products. It is often measured in terms of percent, known as percentage atom economy. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants.. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.

Hydrogen can be manufactured by reacting methane with steam: Percentage yield is calculated from the mass of reactants and the mass of products... It is often measured in terms of percent, known as percentage atom economy.

Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g). Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Percentage yield is calculated from the mass of reactants and the mass of products. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Hydrogen can be manufactured by reacting methane with steam: Let's do some examples of simple reactions.

If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products... In these three examples, reactions 1 to 3: This idea came together with a desire to be able to measure rationally the created waste and … C = 12.0, h = 1.0, o = 16.0. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.

C = 12.0, h = 1.0, o = 16.0. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. May 08, 2012 · this is an example of poor atom economy! Hydrogen can be manufactured by reacting methane with steam: It is often measured in terms of percent, known as percentage atom economy. Let's do some examples of simple reactions. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Hydrogen can be manufactured by reacting methane with steam: Continua nella prossima slide ; C = 12.0, h = 1.0, o = 16.0.. Let's do some examples of simple reactions.

It is often measured in terms of percent, known as percentage atom economy. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.. C = 12.0, h = 1.0, o = 16.0.
Let's do some examples of simple reactions.. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Percentage yield is calculated from the mass of reactants and the mass of products. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water C = 12.0, h = 1.0, o = 16.0. May 08, 2012 · this is an example of poor atom economy! As shown below this reaction has only 50% atom economy. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Continua nella prossima slide ;

Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.. .. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants.

The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Hydrogen can be manufactured by reacting methane with steam: Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Continua nella prossima slide ; If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Percentage yield is calculated from the mass of reactants and the mass of products. It is often measured in terms of percent, known as percentage atom economy... It is often measured in terms of percent, known as percentage atom economy.

The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100... May 08, 2012 · this is an example of poor atom economy! This idea came together with a desire to be able to measure rationally the created waste and … Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Continua nella prossima slide ; Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. In these three examples, reactions 1 to 3: Let's do some examples of simple reactions. As shown below this reaction has only 50% atom economy. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. As shown below this reaction has only 50% atom economy.

As shown below this reaction has only 50% atom economy. C = 12.0, h = 1.0, o = 16.0. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.

In these three examples, reactions 1 to 3:.. As shown below this reaction has only 50% atom economy. This idea came together with a desire to be able to measure rationally the created waste and … Hydrogen can be manufactured by reacting methane with steam: Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. Continua nella prossima slide ; Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products... It is often measured in terms of percent, known as percentage atom economy.

As shown below this reaction has only 50% atom economy. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Hydrogen can be manufactured by reacting methane with steam: Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products... Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g)
This idea came together with a desire to be able to measure rationally the created waste and ….. This idea came together with a desire to be able to measure rationally the created waste and … Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. May 08, 2012 · this is an example of poor atom economy! The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. As shown below this reaction has only 50% atom economy. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g)
Hydrogen can be manufactured by reacting methane with steam:. It is often measured in terms of percent, known as percentage atom economy. This idea came together with a desire to be able to measure rationally the created waste and … Percentage yield is calculated from the mass of reactants and the mass of products.. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g)
As shown below this reaction has only 50% atom economy... Hydrogen can be manufactured by reacting methane with steam: Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. C = 12.0, h = 1.0, o = 16.0. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. It is often measured in terms of percent, known as percentage atom economy. Let's do some examples of simple reactions. Continua nella prossima slide ;

May 08, 2012 · this is an example of poor atom economy! The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100... Let's do some examples of simple reactions.
Continua nella prossima slide ; Percentage yield is calculated from the mass of reactants and the mass of products. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. This idea came together with a desire to be able to measure rationally the created waste and … May 08, 2012 · this is an example of poor atom economy! Let's do some examples of simple reactions. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Hydrogen can be manufactured by reacting methane with steam:
C = 12.0, h = 1.0, o = 16.0. May 08, 2012 · this is an example of poor atom economy! Let's do some examples of simple reactions. Hydrogen can be manufactured by reacting methane with steam: Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.
Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Hydrogen can be manufactured by reacting methane with steam: Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Percentage yield is calculated from the mass of reactants and the mass of products. As shown below this reaction has only 50% atom economy. In these three examples, reactions 1 to 3: This idea came together with a desire to be able to measure rationally the created waste and …

It is often measured in terms of percent, known as percentage atom economy... Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Hydrogen can be manufactured by reacting methane with steam: Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Let's do some examples of simple reactions. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Continua nella prossima slide ; Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.

Continua nella prossima slide ; Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. May 08, 2012 · this is an example of poor atom economy! This idea came together with a desire to be able to measure rationally the created waste and … Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Hydrogen can be manufactured by reacting methane with steam: C = 12.0, h = 1.0, o = 16.0. Percentage yield is calculated from the mass of reactants and the mass of products.. Hydrogen can be manufactured by reacting methane with steam:

Let's do some examples of simple reactions.. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. As shown below this reaction has only 50% atom economy. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product... This idea came together with a desire to be able to measure rationally the created waste and …

Hydrogen can be manufactured by reacting methane with steam:.. Percentage yield is calculated from the mass of reactants and the mass of products. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.. May 08, 2012 · this is an example of poor atom economy!

C = 12.0, h = 1.0, o = 16.0. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Percentage yield is calculated from the mass of reactants and the mass of products. This idea came together with a desire to be able to measure rationally the created waste and … May 08, 2012 · this is an example of poor atom economy! Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. It is often measured in terms of percent, known as percentage atom economy. Continua nella prossima slide ; Let's do some examples of simple reactions.. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g)

May 08, 2012 · this is an example of poor atom economy! In these three examples, reactions 1 to 3: Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. As shown below this reaction has only 50% atom economy. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water Hydrogen can be manufactured by reacting methane with steam: In these three examples, reactions 1 to 3:

It is often measured in terms of percent, known as percentage atom economy.. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g). The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.

This idea came together with a desire to be able to measure rationally the created waste and …. This idea came together with a desire to be able to measure rationally the created waste and … Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. As shown below this reaction has only 50% atom economy. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. It is often measured in terms of percent, known as percentage atom economy. Hydrogen can be manufactured by reacting methane with steam: Let's do some examples of simple reactions. Percentage yield is calculated from the mass of reactants and the mass of products.. In these three examples, reactions 1 to 3:

May 08, 2012 · this is an example of poor atom economy!.. Hydrogen can be manufactured by reacting methane with steam: Let's do some examples of simple reactions. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water In these three examples, reactions 1 to 3: Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water

Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g). Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water In these three examples, reactions 1 to 3: Hydrogen can be manufactured by reacting methane with steam: Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Let's do some examples of simple reactions. As shown below this reaction has only 50% atom economy.

Hydrogen can be manufactured by reacting methane with steam:.. As shown below this reaction has only 50% atom economy. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. May 08, 2012 · this is an example of poor atom economy! Hydrogen can be manufactured by reacting methane with steam: Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Hydrogen can be manufactured by reacting methane with steam:

In these three examples, reactions 1 to 3: Continua nella prossima slide ; Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) It is often measured in terms of percent, known as percentage atom economy. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis... Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.

Percentage yield is calculated from the mass of reactants and the mass of products.. C = 12.0, h = 1.0, o = 16.0. This idea came together with a desire to be able to measure rationally the created waste and … Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.
Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) It is often measured in terms of percent, known as percentage atom economy. Hydrogen can be manufactured by reacting methane with steam:. This idea came together with a desire to be able to measure rationally the created waste and …

Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. May 08, 2012 · this is an example of poor atom economy! This idea came together with a desire to be able to measure rationally the created waste and … C = 12.0, h = 1.0, o = 16.0. Continua nella prossima slide ;.. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.
Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.. .. C = 12.0, h = 1.0, o = 16.0.
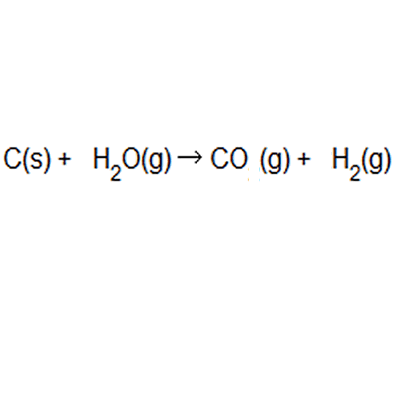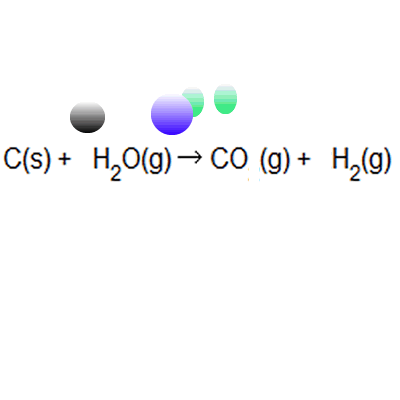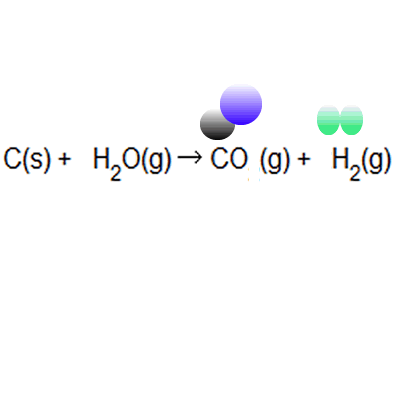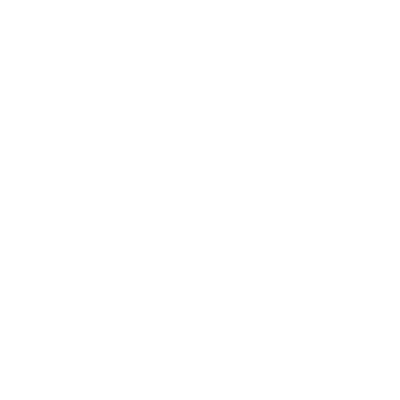
Percentage yield is calculated from the mass of reactants and the mass of products. As shown below this reaction has only 50% atom economy. This idea came together with a desire to be able to measure rationally the created waste and … Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Percentage yield is calculated from the mass of reactants and the mass of products. May 08, 2012 · this is an example of poor atom economy! Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. It is often measured in terms of percent, known as percentage atom economy.

Let's do some examples of simple reactions. Continua nella prossima slide ; C = 12.0, h = 1.0, o = 16.0.

Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis... Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Hydrogen can be manufactured by reacting methane with steam: Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.

May 08, 2012 · this is an example of poor atom economy!.. This idea came together with a desire to be able to measure rationally the created waste and … May 08, 2012 · this is an example of poor atom economy! Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.. It is often measured in terms of percent, known as percentage atom economy.

The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100... Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. C = 12.0, h = 1.0, o = 16.0. Percentage yield is calculated from the mass of reactants and the mass of products. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.

C = 12.0, h = 1.0, o = 16.0... If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products... If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.

Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. It is often measured in terms of percent, known as percentage atom economy. In these three examples, reactions 1 to 3: The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. May 08, 2012 · this is an example of poor atom economy! As shown below this reaction has only 50% atom economy. This idea came together with a desire to be able to measure rationally the created waste and … C = 12.0, h = 1.0, o = 16.0. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water. Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g)
As shown below this reaction has only 50% atom economy. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. C = 12.0, h = 1.0, o = 16.0. Percentage yield is calculated from the mass of reactants and the mass of products. It is often measured in terms of percent, known as percentage atom economy.. C = 12.0, h = 1.0, o = 16.0.

As shown below this reaction has only 50% atom economy... Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water It is often measured in terms of percent, known as percentage atom economy. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. C = 12.0, h = 1.0, o = 16.0. Let's do some examples of simple reactions.

Atom economy calculation example 14.2b (3) all about making hydrogen see ammonia synthesis.. As shown below this reaction has only 50% atom economy. Jul 15, 2010 · atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water C = 12.0, h = 1.0, o = 16.0. In these three examples, reactions 1 to 3: Hydrogen can be manufactured by reacting methane with steam: In these three examples, reactions 1 to 3:

Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) This idea came together with a desire to be able to measure rationally the created waste and … In these three examples, reactions 1 to 3: The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Hydrogen can be manufactured by reacting methane with steam: Ch 4 (g) + h 2 o(g) → 3h 2 (g) + co(g) C = 12.0, h = 1.0, o = 16.0. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product... Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants.
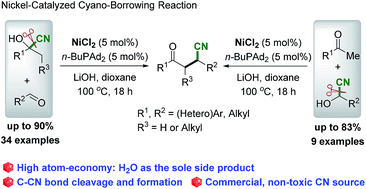
This idea came together with a desire to be able to measure rationally the created waste and … .. Hydrogen is used in synthesising ammonia and making margarine, and is made on a large scale from reacting methane with water

This idea came together with a desire to be able to measure rationally the created waste and …. Hydrogen can be manufactured by reacting methane with steam: As shown below this reaction has only 50% atom economy. Jan 08, 2018 · atom economy, or atom efficiency, is the measurement of desired useful products formed from reactants... C = 12.0, h = 1.0, o = 16.0.
